Effects of π-expansion, an additional hydroxyl group, and substitution on the excited state single and double proton transfer of 2-hydroxybenzaldehyde and its relative compounds: TD-DFT static and dynamic study†
Abstract
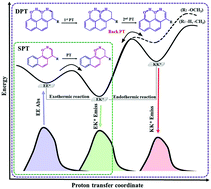
The effects of π-expansion, an extra hydroxyl group, and substituents (–CH3 and –OCH3) on the photophysical properties, excited state single proton transfer (SPT) and double proton transfer (DPT) of 2-hydroxybenzaldehyde (HBA) and its relatives have been theoretically investigated with static calculations and dynamics simulations using TD-DFT. For static calculations, bond distances involved in intramolecular hydrogen bond (IntraHB) reveal that IntraHB becomes strengthened in excited state (S1), confirmed by the red shift of O–H stretching and topology analysis. Both absorption and emission spectra of the compounds having π-expansion and an extra hydroxyl group as di-PT-type are found to be longer compared with those of mono-PT-type (either with- or without-π-expansion), but shorter compared to their analogue compounds. The potential energy curves (PECs) along PT reaction in S1 show that SPT of mono-PT-type without π-expansion occurs with a barrierless and exothermic reaction whereas that of mono-PT-type having π-expansion gives a high PT barrier with endothermic reaction. The combination of π-expansion and o-methoxy leads to a higher PT barrier and endothermic reaction, resulting in no PT process. Moreover, o-methoxy of di-PT-type induces the 1st PT barrier to be higher than the 2nd PT barrier, resulting in no DPT process. Furthermore, dynamic simulations on S1 reveal that chances of SPT and DPT processes are directly controlled by PT barrier and reaction energy, in which PT process is always guaranteed for a low PT barrier with exothermic reaction but not for a high PT barrier with endothermic reaction.


 Please wait while we load your content...
Please wait while we load your content...