Synthesis and anticancer activity of cyclotriphosphazenes functionalized with 4-methyl-7-hydroxycoumarin†
Abstract
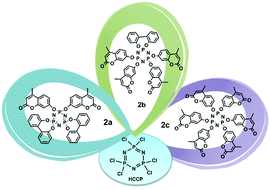
As an important branch of heterocyclic compounds, coumarin and its derivatives with diverse potential biological activities have attracted wide attention and have been applied in medical-related fields. In this article, three cyclotriphosphazene derivatives bearing 4-methyl-7-hydroxycoumarin moieties with the numbers of 2, 4, and 6 were synthesized and characterized and their antitumor activities were investigated. All the new compounds were found to display antitumor activity in vitro against breast cancer cell lines (MCF-7 and 4T1 cells) with the IC50 values in the range of 108.72–188.44 μM and 75.93–154.91 μM, respectively. In contrast, the coumarin monomer displayed the values of 4140 μM and 1640 μM (IC50). Our results suggested that the antitumor activity was significantly enhanced when coumarin was introduced onto the surface of cyclotriphosphazenes, thereby providing potent candidate molecules for pharmaceutical applications.


 Please wait while we load your content...
Please wait while we load your content...