A model compound for pyridinechalcone-based multistate systems. Ring opening-closure as the slowest kinetic step of the multistate†
Abstract
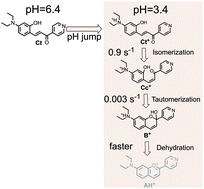
Anthocyanins and related flavylium derivatives exist in aqueous solution as a pH-dependent mole fraction distribution of species (a multistate system) with known biological activity. Introduction of nitrogen heterocycles in the flavylium core can lead to multistates with different constitution and increased activity. Compound 1, a diethylamino derivative of 4-pyridinechalcone, was synthesized and characterized by X-ray crystallography, showing a pH-dependent reaction network similar to anthocyanins and related compounds. The several species present at the equilibrium multistate were fully characterized by 1H NMR and 13C NMR. The thermodynamics and kinetics of the multistate were studied through pH jumps followed by 1H NMR and UV-vis absorption including stopped-flow for the faster kinetic steps. In the parent 4-pyridinechalcone compound, protonation of the pyridine nitrogen for pH < 4 prevents formation of the flavylium cation. In compound 1, the first protonation takes place in the diethylamino substituent and in acidic medium, two new flavylium derivatives, a single (2 < pH < 4) and a double (pH < 1) positively charged species, in equilibrium with protonated hemiketal, cis and trans chalcones, have been characterized. Differently from anthocyanins and related compounds, experimental evidence for an unexpected very slow (0.0003 s−1) ring opening-closure between the hemiketal and the cis-chalcone (tautomerization) was achieved.


 Please wait while we load your content...
Please wait while we load your content...