Molecular structures, and optical and magnetic properties of free-base tetrapyrazinoporphyrazine in various reduction states†
Abstract
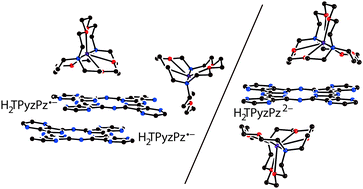
Reduction of free-base tetrapyrazinoporphyrazine (H2TPyzPz) under different experimental conditions allowed us to obtain radical anion salt {cryptand[2.2.2](K+)}2(H2TPyzPz˙−)2·3C6H4Cl2 (1), and dianion salts {cryptand[2.2.2](K+)}2(H2TPyzPz2−)·C6H4Cl2 (2) and {cryptand[2.2.2](K+)}2(H2TPyzPz2−)·[CpFe(CO)2]2·3C6H4Cl2 (3) in the form of single crystals. The molecular structure, and optical and magnetic properties of free-base tetrapyrazinoporphyrazine in various reduction states were studied for the first time. Reduction of the macrocycle is accompanied by the appearance of alternation of the C–Nmeso bonds, blue shift of both the Soret and Q-bands and the appearance of new bands in the NIR range. Salt 1 contained paramagnetic H2TPyzPz˙− radical anions half of which formed face-to-face type dimers with very effective π–π interaction, whereas the rest were magnetically isolated from the other H2TPyzPz˙− radical anions. Strong antiferromagnetic coupling of spins with a magnetic exchange interaction of J/kB = −140 K was observed in these dimers. In 2 and 3, diamagnetic dianions H2TPyzPz2− were completely isolated.


 Please wait while we load your content...
Please wait while we load your content...