High energy density and high working voltage of a quasi-solid-state supercapacitor with a redox-active ionic liquid added gel polymer electrolyte†
Abstract
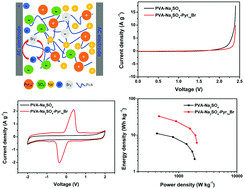
To increase the energy density of quasi-solid-state supercapacitors, a redox-active gel polymer electrolyte (GPE) was prepared by evaporating the excess water in a neutral gel that consists of polyvinyl alcohol (PVA), Na2SO4 and the ionic liquid (IL) N-butyl-N-methylpyrrolidinium bromide (Pyr14Br). The influence of IL Pyr14Br on the ionic conductivity of GPE was investigated. The maximum ionic conductivity of PVA–Na2SO4–Pyr14Br GPE can reach 27.1 mS cm−1. The optimized GPE was assembled with two activated carbon electrodes into a quasi-solid-state supercapacitor. The electrochemical performances of this supercapacitor were evaluated by cyclic voltammetry, galvanostatic charge/discharge, electrochemical impedance spectroscopy and self-discharge measurements. The assembled supercapacitor exhibits a high energy density of 33.0 W h kg−1, which is due to the wide working voltage (2.0 V) as a result of the strong solvation of Na+ cations and SO42− anions and the production of an additional pseudocapacitive contribution from the Br−/Br3− redox reaction at the electrolyte/electrode interface. This supercapacitor exhibits outstanding cyclic stability with an 81.0% capacitance retention ratio after 8000 charge/discharge cycles. Moreover, this supercapacitor presents good self-discharge behavior.


 Please wait while we load your content...
Please wait while we load your content...