Cooperation and competition of hydrogen and halogen bonds in 2D self-assembled nanostructures based on bromine substituted coumarins†
Abstract
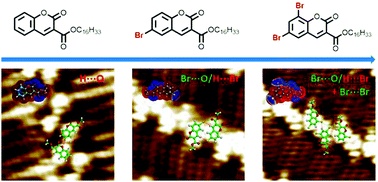
Three coumarin derivatives (Co16, 6-Br-Co16 and 6,8-Br-Co16) with ester, ether, and carbonyl groups and different numbers of bromine substituents on the coumarin cores were synthesized. Their two-dimensional (2D) self-assemblies were probed by scanning tunneling microscopy (STM) at the liquid–solid interface. The Co16 molecules formed a zigzag lamellar pattern on the HOPG surface through intermolecular H⋯O hydrogen bonds. The 6-Br-Co16 molecule with one Br substituent was found to form a uniform zigzag linear pattern, owing to intermolecular Br⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (ester groups) halogen bonds and H⋯Br hydrogen bonds. The dibromo-substituted 6,8-Br-Co16 molecule could form a dislocated linear pattern, in which the molecules are linked through the Br⋯O
C (ester groups) halogen bonds and H⋯Br hydrogen bonds. The dibromo-substituted 6,8-Br-Co16 molecule could form a dislocated linear pattern, in which the molecules are linked through the Br⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (ester group) halogen bond, and Br⋯Br and H⋯Br bonds. The STM results reveal that the introduction of the Br atom actuates the formation of the Br⋯O
C (ester group) halogen bond, and Br⋯Br and H⋯Br bonds. The STM results reveal that the introduction of the Br atom actuates the formation of the Br⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond instead of the H⋯O
C bond instead of the H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, which is the key dominant force to form the different 2D adlayers. At the same time, the cooperation of H⋯Br hydrogen bonds leads to the stability of the nanostructures. DFT calculations were performed to unravel the cooperation and competition mechanism for the formation of halogen bonds and hydrogen bonds in 2D molecular self-assembly.
C bond, which is the key dominant force to form the different 2D adlayers. At the same time, the cooperation of H⋯Br hydrogen bonds leads to the stability of the nanostructures. DFT calculations were performed to unravel the cooperation and competition mechanism for the formation of halogen bonds and hydrogen bonds in 2D molecular self-assembly.


 Please wait while we load your content...
Please wait while we load your content...