A novel methodology for the synthesis of condensed selenium heterocycles based on the annulation and annulation–methoxylation reactions of selenium dihalides†
Abstract
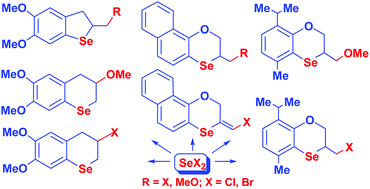
A novel methodology to accelerate annulation reactions leading to condensed selenium heterocycles was developed. The reactions of selenium dihalides with methyleugenol, allyl thymyl ether, allyl 1-naphthyl and 1-naphthyl propargyl ethers were carried out in the presence of alcohols, which considerably accelerated the annulation reactions. In solvent systems CH2Cl2/MeOH or CHCl3/MeOH, the reactions proceeded as annulation–methoxylation affording condensed methoxylated heterocycles. In the presence of isopropanol, the reactions were not accompanied by alkoxylation giving condensed halogen-containing products. The annulation reaction of selenium dihalides with 1-naphthyl propargyl ether included stereoselective anti-addition to the triple bond. The efficient selective synthesis of the first representatives of novel families of condensed selenium heterocycles with promising biological activity was developed.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...