The azide–alkyne cycloaddition catalysed by transition metal oxide nanoparticles†
Abstract
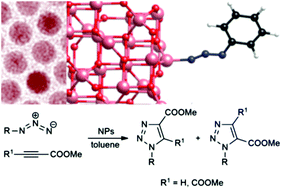
Colloidal nanoparticles of Earth-abundant, first-row transition metal oxides and sulfide, namely magnetite (Fe3O4), manganese and cobalt ferrite, (MnFe2O4, CoFe2O4), manganese(II) oxide (MnO) and sulfide (α-MnS), were used as catalysts in the cycloaddition between azides and methyl propiolate. The presence of these nanoparticles allowed us to carry out the cycloadditions under milder conditions and with a regioselectivity comparable to the classic “metal-free” thermal processes. Ferrite nanoparticles gave higher conversion than MnO and α-MnS nanoparticles. The feasibility of the cycloaddition onto 1,2-disubstituted acetylenes was also proved. Ferrite nanocatalysts could be magnetically recovered and reused without significant loss of catalytic activity. Density functional theory (DFT) calculations support a mechanistic hypothesis that attributes the increased cycloaddition rate to the adsorption of the azide onto to the nanocatalyst surface.


 Please wait while we load your content...
Please wait while we load your content...