DFT study on the redox behavior of two dioxovanadium(v) complexes with N2O donor Schiff base ligands and their use in catalytic oxidation of ortho-aminophenol†
Abstract
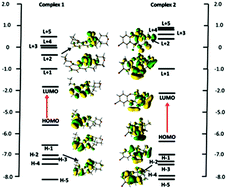
Two vanadium(V) Schiff base complexes, [VO2L1] (1) and [VO2L2] (2) [where HL1 = 2-((2-morpholinoethylimino)methyl)-6-ethoxyphenol and HL2 = 2-((2-(dimethylamino)ethylimino)methyl)-4-bromophenol], were synthesized and characterized by elemental and spectral analysis. The structures of both complexes were confirmed by single-crystal X-ray diffraction studies. To investigate the redox properties of the complexes, electrochemical measurements were performed in solution. Density functional theory (DFT) calculations have been performed using hybrid functional PBE0. The DFT optimized results were found to agree well with the experimental results. Topological features derived from the Bader's theory of atoms in molecules (AIM) approach have also been applied. Phenoxazinone synthase like activities (conversion of o-aminophenol to 2-aminophenoxazine-3-one) of both complexes were investigated spectrophotometrically.


 Please wait while we load your content...
Please wait while we load your content...