Aminonitrones as highly reactive bifunctional synthons. An expedient one-pot route to 5-amino-1,2,4-triazoles and 5-amino-1,2,4-oxadiazoles – potential antimicrobials targeting multi-drug resistant bacteria†
Abstract
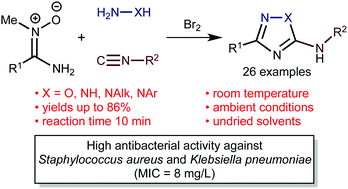
The developed one-pot protocol to 5-amino-1,2,4-triazoles or 5-amino-1,2,4-oxadiazoles includes an interplay between aminonitrones R1C(NH2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+(Me)O− (R1 = Alk, Ar, Het), isocyanides R2NC (R2 = Alk, Ar), Br2, and hydrazines (for the triazoles) or hydroxylamine (for the oxadiazoles). This formally four-component reaction, involving aminonitrones, isocyanides, bromine, and N-nucleophiles, proceeds very rapidly under mild conditions (10 min, 20–25 °C), and is insensitive to moisture and air (in undried CHCl3–MeOH, in air) and it gives the heterocyclic systems in good yields (up to 86%; 26 examples). The reaction scope includes aromatic-, heteroaromatic-, and aliphatic aminonitrones and also aliphatic- and aromatic isocyanides. Results of DFT calculations (M06-2X/6-311+G(d,p) level of theory) indicate that the O-nucleophilic center of bifunctional aminonitrones is more reactive than the N center; it first reacts with in situ generated R2NCBr2 to grant 2-methyl-1,2,4-oxadiazolium salts, which are then converted to the target heterocyclic systems upon treatment with hydrazines or hydroxylamine. The nature and strength of the intramolecular hydrogen bonds N–H⋯N and O–H⋯N, which significantly contribute to the total energies of different transition states and products of the nucleophilic substitution, were studied theoretically using the topological analysis of the electron density distribution within the framework of Bader's theory (QTAIM method). Several new 5-amino-3-aryl-1,2,4-triazoles and -1,2,4-oxadiazoles exhibit high antibacterial activity against multidrug-resistant bacteria strains such as Staphylococcus aureus and Klebsiella pneumoniae (MIC = 8 mg L−1).
N+(Me)O− (R1 = Alk, Ar, Het), isocyanides R2NC (R2 = Alk, Ar), Br2, and hydrazines (for the triazoles) or hydroxylamine (for the oxadiazoles). This formally four-component reaction, involving aminonitrones, isocyanides, bromine, and N-nucleophiles, proceeds very rapidly under mild conditions (10 min, 20–25 °C), and is insensitive to moisture and air (in undried CHCl3–MeOH, in air) and it gives the heterocyclic systems in good yields (up to 86%; 26 examples). The reaction scope includes aromatic-, heteroaromatic-, and aliphatic aminonitrones and also aliphatic- and aromatic isocyanides. Results of DFT calculations (M06-2X/6-311+G(d,p) level of theory) indicate that the O-nucleophilic center of bifunctional aminonitrones is more reactive than the N center; it first reacts with in situ generated R2NCBr2 to grant 2-methyl-1,2,4-oxadiazolium salts, which are then converted to the target heterocyclic systems upon treatment with hydrazines or hydroxylamine. The nature and strength of the intramolecular hydrogen bonds N–H⋯N and O–H⋯N, which significantly contribute to the total energies of different transition states and products of the nucleophilic substitution, were studied theoretically using the topological analysis of the electron density distribution within the framework of Bader's theory (QTAIM method). Several new 5-amino-3-aryl-1,2,4-triazoles and -1,2,4-oxadiazoles exhibit high antibacterial activity against multidrug-resistant bacteria strains such as Staphylococcus aureus and Klebsiella pneumoniae (MIC = 8 mg L−1).


 Please wait while we load your content...
Please wait while we load your content...