An insight into the outer- and inner-sphere electrochemistry of oxygenated single-walled carbon nanohorns (o-SWCNHs)†
Abstract
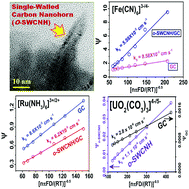
The extremely large surface areas as well as connecting nanopores of single-walled carbon nanohorn (SWCNH) aggregates have been tested, for the first time to the best of our knowledge, as metal-free, stable and cheap electrocatalysts for heterogeneous electron transfer reactions involving inorganic redox couples, including f-block elements such as actinides. From systematic studies of heterogeneous electron transfer reactions, the electrochemically reversible outer-sphere heterogeneous surface insensitive electron transfer reaction involving [Ru(NH3)6]3+/[Ru(NH3)6]2+ and the electrochemically near-to-reversible inner-sphere heterogeneous surface sensitive electron transfer reaction involving [Fe(CN)6]3−/[Fe(CN)6]4− on oxygenated SWCNHs (i.e., o-SWCNHs) have been compared to heterogeneous electron transfer involving the quasi-reversible [U(VI)O2(CO3)3]4−/[U(V)O2(CO3)3]5− redox reaction on o-SWCNHs. It was evident that the oxygen containing functional groups of o-SWCNH could catalyze the electron transfer process between uranium species in solution and the working electrode happened by following surface-sensitive inner-sphere electron transfer mechanism. Furthermore, the electrochemical stability, repeatability and reproducibility of the o-SWCNH modified glassy carbon electrode were found to be analytically acceptable for studying the electrochemistry of uranium in alkaline solutions with high ionic strength.


 Please wait while we load your content...
Please wait while we load your content...