Hydroformylation of unsaturated esters and 2,3-dihydrofuran under solventless conditions at room temperature catalysed by rhodium N-pyrrolyl phosphine catalysts†
Abstract
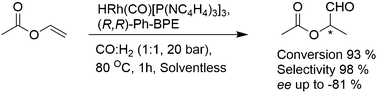
Rhodium complexes of the type HRh(CO)L3 (where L is an N-pyrrolyl phosphine, such as P(NC4H4)3, PPh(NC4H4)2, or PPh2(NC4H4)) were applied in the hydroformylation of less reactive unsaturated substrates, namely allyl acetate, butyl acrylate, methyl acrylate, 2,3-dihydrofuran and vinyl acetate. Even at room temperature, these catalysts enabled complete substrate conversion and high chemoselectivity towards the corresponding aldehydes. High conversion of vinyl acetate (88% in 6 h) to the branched aldehyde was obtained with HRh(CO)[P(NC4H4)3]3 at 25 °C. An increase of the turnover frequency, TOF, up to 2000 mol mol−1 h−1 was achieved in this reaction under 20 bar of syngas (H2/CO = 1) at 80 °C. The introduction of chiral phosphines, BINAP or Ph-BPE, to this system resulted in the production of a branched aldehyde with enantioselectivity, ee, up to 44 and 81%, respectively. High activity combined with high enantioselectivity was achieved due to the formation of the mixed rhodium hydrides HRh(CO)[P(NC4H4)3](BINAP) and HRh(CO)[P(NC4H4)3](Ph-BPE), identified by the NMR method.


 Please wait while we load your content...
Please wait while we load your content...