Exploring solvent dependent catecholase activity in transition metal complexes: an experimental and theoretical approach†
Abstract
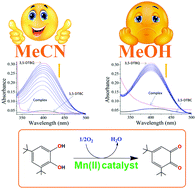
In contrast to polynuclear complex models for biomimetic activity, a series of mononuclear complexes are designed in this work in order to assess their catecholase activity and kinetics. Four new complexes, namely, [Mn(pdmH2)(Phen)Cl]Cl·H2O (1), [Mn(pmmH)2(SCN)2] (2), [Ni(pmmH)2(SCN)2] (3) and [Zn(phen)3][(pdm)]·11H2O (4) utilizing an amino alcohol ligand, pyridine-2,6-dimethanol (pdmH2) or 2-pyridinemonomethanol (pmmH), and an auxiliary ligand, 1,10-phenanthroline (phen) or thiocyanate (SCN−), are synthesized. The complexes are characterized by elemental analysis, FTIR, UV-visible, EPR, fluorescence (solution and solid state), Hirshfeld surface analysis, magnetic, single crystal X-ray and DFT/TD-DFT studies. X-ray structures confirm the geometry around the M(II) ions to be octahedral in all the complexes. In 1–3, the primary aminoalcohol ligand binds to a metal ion in a neutral (pdmH2 or pmmH) mode while 4 exists as a cation–anion type complex where a deprotonated ligand (pdm2−) is present in the outer sphere having no coordination to the metal. The Zn(II) complex also shows remarkable luminescence in the solid state photoluminescence spectrum. Variable temperature magnetic studies show the presence of antiferromagnetic exchange in 1–3 (θ = −2.8, −1.7 and −5.2, respectively) with the observation of anisotropy (D = 4.0 and E = 3.4) in 3. DFT/TD-DFT results provide ample information regarding the structures, spin densities, charge distribution, and electronic spectra along with the transitions. The spin density values, ρ = 4.793, 4.792 and 1.676, confirm the presence of five, five and two unpaired electrons on the metal d-orbitals of 1, 2 and 3, respectively. Interestingly, solvent dependent catecholase activity has been observed for the first time in mononuclear Mn(II) complexes (1 and 2) with Kcat = 2602.8 h−1 (acetonitrile), 1490.4 h−1 (methanol) for 1, and 1083.6 h−1 (acetonitrile), 806.4 h−1 (methanol) for 2. This can be rationalized in terms of the coordinating powers of the solvent, i.e., DMSO > MeOH > MeCN. Further, the order of activity in 1 and 2 (i.e., 1 > 2) and inactivity of 3 and 4 are very well corroborated by DFT which ascertains the highest charge contribution on the metal in 1 [33% on HOMO and 2% on LUMO of Mn(II)] resulting in the formation of the most stable metal–substrate adduct, thus enhancing the activity.


 Please wait while we load your content...
Please wait while we load your content...