Investigation of the kinetics and mechanism of Z-scheme Ag3PO4/WO3 p–n junction photocatalysts with enhanced removal efficiency for RhB†
Abstract
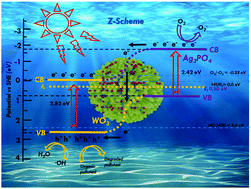
Semiconductor-based photocatalysis attracts wide attention owing to its ability to directly utilize solar energy for the production of solar fuels. In particular, the construction of Z-scheme heterojunctions is demonstrated to benefit the photocatalytic performance because of the separation and transfer of the photogenerated charge carriers and well-preserved strong redox ability. In this work, a series of Z-scheme Ag3PO4/WO3 p–n junction photocatalysts were prepared employing a facile impregnation method. The photocatalysts (Ag3PO4/WO3 p–n junction) presented enhanced photo-degradation efficiency; for example, with AW-45, 99.9% degradation could be obtained for RhB (30 ppm) on irradiation (λ > 420 nm, 350 W xenon lamp) for 7 min, which is a great improvement compared with the individual constituents. The degradation rate for AW-45 was 27 times higher than that of a commercial powder (P25). The apparent kinetic rate of catalytic degradation was obtained according to the results from the degradation process. The mechanism for electron transfer between Ag3PO4 and WO3 was also investigated. The reduction and oxidation processes concentrated on the CB (conducting band) of Ag3PO4 and the VB (valence band) of WO3, resulting in promoting the mechanism for photocatalytic degradation in the Z-scheme Ag3PO4/WO3 p–n junction photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...