Investigation of catalytic activity and mechanism for RhB degradation by LaMnO3 perovskites prepared via the citric acid method
Abstract
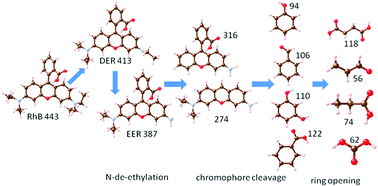
LaMnO3 nanoparticles were synthesized by the citric acid complex sol–gel method and used to degrade rhodamine B (RhB) in aqueous solution. We found that without adding additional oxidants to the reaction system or light irradiation, LaMnO3 exhibits an excellent degradation ability of RhB under highly acidic conditions and achieves about 98.0% degradation efficiency. This result showed that LaMnO3 itself provides an effective oxidative system to degrade RhB. Furthermore, based on the intermediates detected by ultraviolet-visible spectrophotometry (UV-vis) and liquid chromatography mass spectrometry (LC-MS), a reasonable degradation pathway of RhB was proposed, including N-de-ethylation, chromophore cleavage, ring opening and mineralization. LaMnO3 can maintain the perovskite structure in the reaction system, and abundant vacancies of LaMnO3 promote the generation of active oxygen species. Furthermore, electron transfer between Mn4+ and Mn3+ in LaMnO3 is the key to the degradation of organic matter in this reaction system.


 Please wait while we load your content...
Please wait while we load your content...