One-pot achievement of MnO2/Fe2O3 nanocomposites for the oxygen reduction reaction with enhanced catalytic activity†
Abstract
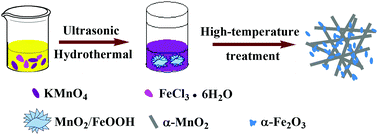
MnO2/Fe2O3 nanocomposites were achieved via using KMnO4 and FeCl3 as starting materials in aqueous solution followed by hydrothermal and high-temperature treatment processes. The hydrolysis of FeCl3 produces H+ ions to react with KMnO4 to produce MnO2, which further accelerates the hydrolysis process. The as-synthesized MnO2/Fe2O3 nanocomposites present high oxygen reduction reaction activity with an onset and half-wave potential of 0.953 and 0.859 V vs. RHE, respectively, and a limited current density of 4.875 mA cm−2 at 0.565 V, which are comparable to those of the commercial Pt/C catalyst. The excellent catalytic performance is considered to originate from the high Mn3+ content and the related oxygen vacancies. Furthermore, the unsatisfactory stability of the MnO2/Fe2O3 nanocomposites can be solved by coating with a layer of polyaniline-derived carbon.


 Please wait while we load your content...
Please wait while we load your content...