Rapid and selective colorimetric sensing of Au3+ ions based on galvanic displacement of silver nanoparticles†
Abstract
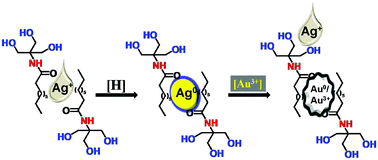
This paper describes a simple, efficient, and selective colorimetric sensor for Au3+ ions based on N-deconyltromethamine functionalized silver nanoparticles (AgNPs). Spectrophotometric study revealed that the surface plasmon resonance (SPR) maximum of AgNPs at 415 nm decreased selectively with the addition of Au3+ ions, but in 60 min, a new SPR maximum of Au0 was observed at 540 nm. The result suggests that Au3+ ions have been reduced by a galvanic displacement reaction. An electrochemical study conducted with an AgNP modified carbon paste electrode revealed that the addition of Au3+ ions increases the open circuit potential, which supports the displacement of AgNPs and the formation of an interface between metallic Au and Au3+ solution. The naked eye detection of Au3+ ions was monitored through a colour change from yellow to colorless and the detection limit (DL) was 12 μM, whereas the spectrophotometric DL was 3.53 μM. The proposed colorimetric probe has been successfully applied for the detection of Au3+ solution in tap water, river water, and sewage water with low interference and good recovery. The simplicity of the described sensor may be useful in the future to develop methods for geochemical prospecting.


 Please wait while we load your content...
Please wait while we load your content...