Highly efficient COx-free hydrogen evolution activity on rod Fe2N catalysts for ammonia decomposition†
Abstract
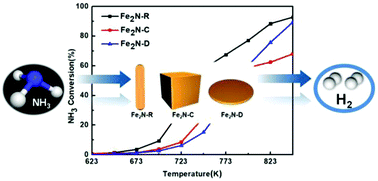
Ammonia decomposition is a critical method for COx-free hydrogen production, and many metal nitrides have been studied as excellent catalysts for NH3 decomposition recently. Here, Fe2N catalysts with rod, cubical and discoidal structures were prepared via nitrogenization of the corresponding morphologies of Fe2O3 precursors, which were obtained by a hydrothermal synthesis method. These Fe2N catalysts were characterized by XRD, TEM, BET, XPS and Mössbauer spectroscopy, and the results showed that the morphologies of Fe2O3 precursors had a significant effect on the crystallite size and yield of the nitride phase of Fe2N catalysts. The rod Fe2N had a small crystallite size and a high relative nitrogen content among the three samples. The catalytic activity for hydrogen production via ammonia decomposition was measured using a micro-fixed bed reactor under conditions of conventional pressure and a GHSV of 6000 mL gcat−1 h−1. The rod Fe2N exhibited a higher NH3 conversion of 90% at 823 K compared with Fe2N derived from cubical and discoidal Fe2O3, and maintained high stability for 40 h.


 Please wait while we load your content...
Please wait while we load your content...