ZnO-NP assisted synthesis of fluorescent β-carboline C-1 tethered benzimidazole/benzothiazole/benzoxazole derivatives and assessment of their photophysical properties†
Abstract
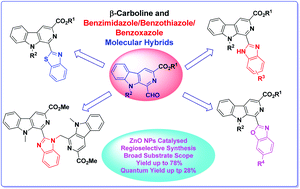
A facile transformation of 1-formyl β-carboline into fluorescent β-carboline C-1 tethered benzazole derivatives is described under the catalysis of ZnO nanoparticles. The reaction proceeded with the reaction of 1-formyl β-carboline and substituted o-diaminobenzene/2-aminobenzenethiol/2-aminophenol, which results in formation of a Schiff base, followed by an intramolecular cylization reaction to generate β-carboline linked benzimidazole, benzothiazole and benzoxazole derivatives. This appraoch displayed a wide substrate scope and high regioselectivity to yield the desired products in moderate to good yields. The photophysical properties of the synthesized derivatives were also evaluated and they exhibited excellent fluorescence properties. Among these β-carboline substituted azoles, the benzothiazole derivative displayed the maximum quantum yield (ΦF up to 28%).


 Please wait while we load your content...
Please wait while we load your content...