Boosting acetone oxidation efficiency over MnO2 nanorods by tailoring crystal phases†
Abstract
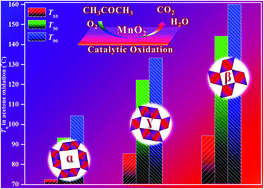
Developing metal oxides with tailored crystal phases has become a research hotspot in environmental catalysis. In this work, three kinds of MnO2 nanorods with different crystal phases, e.g. α-, β- and γ-MnO2, have been prepared by a one-pot hydrothermal method with goals to explore their crystal-phase dependent catalytic performances for acetone elimination. The results attest that α-MnO2 gave the optimal acetone oxidation activity as compared with β- and γ-MnO2, completely achieving 100% acetone conversion and 100% CO2 selectivity at 120 °C under the reaction conditions of acetone concentration = 1000 ppm, 20 vol% O2/N2 and WHSV = 90 000 mL gcat−1 h−1. This superior activity of α-MnO2 mainly originated from its unique crystal phase that resulted in the synergistic effect by combining the largest crystal tunnel size, the highly enhanced chemical nature originating from more Mn4+ cations, the highly improved low-temperature redox properties and the weakest Mn–O bond strength. Meanwhile, three kinds of MnO2 nanorods also demonstrated strong long-term stability and good water tolerance for acetone elimination, showing good potential in practical applications.


 Please wait while we load your content...
Please wait while we load your content...