Synthesis, characterization, and cytotoxic and antimicrobial activities of mixed-ligand hydrazone complexes of variable valence VOz+ (z = 2, 3)†
Abstract
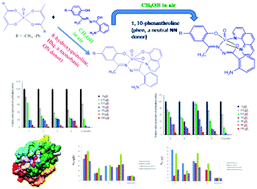
Two sets of mixed-ligand complexes were synthesized and characterized, namely, [VIVO(L1–4)(phen)] (1–4) and [VVO(L1–4)(hq)] (5–8) incorporating 2-aminobenzoylhydrazone of 2-hydroxyacetophenone (H2L1), 2-hydroxy-5-methylacetophenone (H2L2), 2-hydroxy-5-methoxyacetophenone (H2L3) and 5-chloro-2-hydroxyacetophenone (H2L4) as primary ligands together with 1,10-phenanthroline (phen) and 8-hydroxyquinoline (Hhq) as co-ligands. The complexes were characterized by elemental analyses, magnetic susceptibility measurements and various spectroscopic techniques. The structures of complexes 2, 5 and 8 were determined by single crystal X-ray diffractometry. This study indicates that the co-ligands have remarkable effects on the selective stabilization of the oxidation state of vanadium because the neutral N,N donor phen ligand stabilizes the +IV state, while the monobasic O−,N donor hq− ligand stabilizes the +V state. Substituents on the aryloxy ring also had significant effects on the electronic properties of vanadium in the resulting complexes. The E1/2 values of all the complexes and the λmax values for the LMCT transitions of pentavalent complexes 5–8 exhibited linear relationships with the Hammett parameter of the substituent. The complexes exhibited promising cytotoxic activity against lung cancer cells. Interestingly, complexes 2, 3 and 4 (with IC50 values of ca. 2.5 μM) exhibited cytotoxic activity comparable to that found for the widely used cisplatin (also with an IC50 value of 2.5 μM). Nuclear staining experiments suggest that the complexes kill the cells through apoptosis, which is further substantiated by molecular docking studies. These complexes also exhibited potential antimicrobial activity against Escherichia coli, Bacillus subtilis, Staphylococcus aureus and Salmonella typhimurium.


 Please wait while we load your content...
Please wait while we load your content...