Synthesis and facile structure-adjusting of Pd–Pt nanocrystal electrocatalysts with improved activity for ethanol oxidation reaction
Abstract
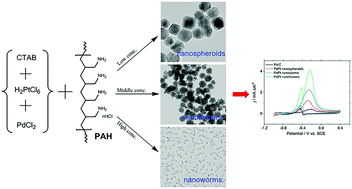
Herein, we report a surfactant-controlled strategy for synthesizing Pd–Pt bimetallic nanocrystals (NCs) with different morphologies via a high-yield and one-pot synthesis method in an aqueous solution for catalyzing the ethanol oxidation reaction. Polyallylamine hydrochloride (PAH) was utilized to modulate the reduction kinetics to obtain diverse morphologies. By simply changing the concentration of surface controller PAH in the original growth solution, three different shapes of Pd–Pt NCs (nanospheroids, nanoflowers, and nanoworms) could be prepared by using H2PtCl6 and PdCl2 as metal precursors and CTAB as a stabilizer. Compared with nanoworms and nanospheroids, the as-prepared nanoflowers exhibited the highest electrocatalytic activity and possessed Pd–Pt core–shell nanostructures. The important role of PAH was confirmed to tune the reduction kinetics, resulting in the distinct morphologies of Pd–Pt NCs. To the best of our knowledge, this is the first report in which Pd–Pt NCs with PAH-controlled structures are prepared in a one-pot aqueous phase by adjusting the sequential reduction kinetics. Furthermore, these Pd–Pt NCs exhibited superior catalytic activity for the ethanol electrooxidation in comparison with that of the commercial Pd/C catalyst.


 Please wait while we load your content...
Please wait while we load your content...