Alkaline earth ion exchange study of pure silica LTA zeolites using periodic first-principles calculations†
Abstract
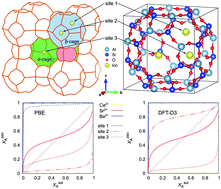
Experiments show that zeolites, such as Linde Type A (LTA), are promising materials for radioactive decontamination processes. In this work, the thermodynamic properties associated with alkaline earth ion (Ca2+, Sr2+ and Ba2+) exchange in pure silica LTA was studied using periodic first-principles calculations. The adsorption energies of alkaline earth ions compared with that of Na+ were investigated. The driving forces for alkaline earth exchange and related isotherms were calculated, and analyzed as a function of the electron chemical potential. The results demonstrate that Na+ in a pure silica LTA can completely be removed by Ba2+ and almost completely be removed by Sr2+ from a stream, but cannot be effectively exchanged by Ca2+. We also showed that electron-donating dopants should suppress Sr2+ and Ba2+ ion exchange, and that there is a substantial preference for incorporating Ba2+ over Sr2+. Lastly, the substantial difference between the adsorption energies of the ions in an assumed vacuum and those computed in water suggests that a solvation model is needed for accurate representation of ion adsorption in an LTA zeolite.


 Please wait while we load your content...
Please wait while we load your content...
