Synthesis of novel magnetic ionic liquids as high efficiency catalysts for extraction-catalytic oxidative desulfurization in fuel oil
Abstract
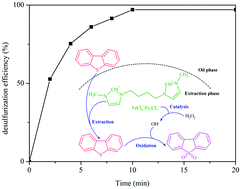
Magnetic ionic liquids (MILs) exhibit high catalytic activity and selectivity in extractive and catalytic oxidative desulfurization (ECODS). In this study, a series of novel MILs, [Cn(mim)2]Cl2/mFeCl3 (n = 2, 4 or 6 and m = 1, 2 or 3), were prepared and introduced in ECODS of model oil with H2O2 as the oxidant. The catalytic performance of the prepared catalysts under different reaction parameters and their recyclability were evaluated. The results indicated that the acidity and hydrophobicity of the catalysts are the main factors affecting the oxidation/extraction process. [C4(mim)2]Cl2/2FeCl3 exhibited the highest desulfurization efficiency such that 97.07% of DBT removal was obtained in 10 min under optimum conditions. Moreover, [C4(mim)2]Cl2/2FeCl3 could be easily separated from the oil phase by an external magnetic field due to its strong magnetism and could be recycled 7 times with no significant decline in its activity after regeneration, thus exhibiting excellent recyclability. Finally, the desulfurization product was determined to be DBTO2 by FT-IR analysis and the hydroxyl radicals produced by Fe3+ and H2O2 played an important role in the desulfurization process. This study provides a new type of catalyst with high activity, selectivity, and stability for fuel desulfurization, which has potential industrial application prospects.


 Please wait while we load your content...
Please wait while we load your content...