Excess adsorption of acetonitrile and water on MIL-100(Fe) and its potential application in mixed-mode chromatography†
Abstract
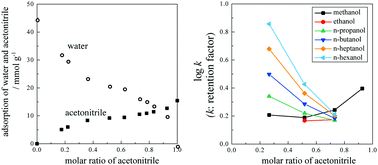
The excess adsorption isotherm of acetonitrile (ACN) on a metal–organic framework constructed from Fe(III) and 1,3,5-benzenetricarboxylic acid (MIL-100(Fe)) in ACN/water solution was measured and converted to adsorption isotherms of ACN and water. Excess ACN was adsorbed on MIL-100(Fe) when the volume fraction of ACN (xA) was smaller than ∼0.55, while excess water was adsorbed when xA ≥ 0.55. Water should adsorb on Fe(III), while ACN has hydrophobic interactions with the aromatic rings of 1,3,5-benzenetricarboxylic acid. The adsorption isotherms indicate that water and ACN adsorption were comparable, indicating that MIL-100(Fe) may be applicable as the stationary phase in mixed-mode liquid chromatography. Investigation of solute retention revealed that the order of solute retention is switched depending on xA. At xA ≤ 0.7, solute retention may be dominated by hydrophobic interactions between the solute and the aromatic rings of the MIL-100(Fe) framework. At xA ≥ 0.7, solute retention cannot be explained simply by the normal-phase or reversed-phase mode. Interaction with Fe(III) should be taken into account in addition to hydrophobic interactions. Therefore, MIL-100(Fe) is a potential candidate for the stationary phase in mixed mode liquid chromatography.


 Please wait while we load your content...
Please wait while we load your content...