Copper(ii) accelerated azide–alkyne cycloaddition reaction using mercaptopyridine-based triazole ligands†
Abstract
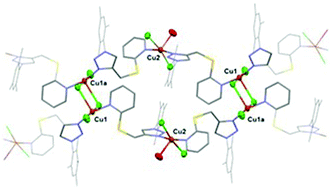
We report the preparation and full characterization of a series of mercapto pyridine-functionalized 1,2,3-triazoles and their use as ligands for the preparation of copper(II) complexes. Complex 1 supported by a 2-mercaptopyridine functionalized triazole (A) and featuring a polymeric structure with the coordination of CuCl2 centers at both the triazole and pyridine fragments, displays a highly effcient click-type catalytic performance in alcoholic solvents without the need for an external reducing agent. Experimental results suggest that the copper(II) species undergo reduction to catalytic copper(I) via alcohol oxidation during an induction period. The scope of the click reaction is broad including the high yielding synthesis of a series of mono, bis, and tris-triazoles, using microwave radiation under low catalyst loadings.


 Please wait while we load your content...
Please wait while we load your content...