The use of enaminones and enamines as effective synthons for MSA-catalyzed regioselective synthesis of 1,3,4-tri- and 1,3,4,5-tetrasubstituted pyrazoles†
Abstract
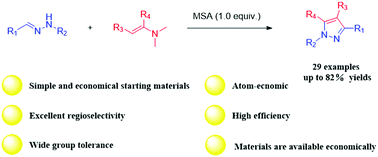
In the present work, an efficient regioselective synthesis of 1,3,4-tri- and 1,3,4,5-tetrasubstituted pyrazoles via a methanesulfonic acid (MSA)-catalyzed reaction of hydrazones with enaminones or enamines is reported. Mechanistically, the formation of the title compounds involves the [2+3] cycloaddition of hydrazones with enaminones or enamines followed by aromatization with acid and oxygen. This convenient method under mild conditions with various hydrazones, enaminones, and enamines was well-tolerated to afford products in good to excellent yields. Compared with the literature methods, this strategy has advantages such as materials that are available economically, metal-free catalysis, excellent regioselectivity, and high efficiency.


 Please wait while we load your content...
Please wait while we load your content...