Design, synthesis, characterization and evaluation of the anticancer activity of water-soluble half-sandwich ruthenium(ii) arene halido complexes†
Abstract
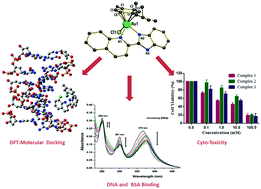
The synthesis, detailed characterization, and investigation of the DNA- and BSA-binding affinity and cytotoxicity of three half-sandwich Ru(II) arene chlorido complexes (1–3) are reported. Specifically, organometallic complexes [(η6-p-cymene)(L1)RuCl]Cl (1), [(η6-p-cymene)(L2)RuCl]Cl (2) and [(η6-p-cymene)(L3)RuCl]Cl (3) [L1 = 2-(1H-benzo[d]imidazol-2-yl)quinolone, L2 = 2-(quinolin-2-yl)benzo[d]oxazole, and L3 = 2-(quinolin-2-yl)benzo[d]thiazole] were synthesized using [((η6-p-cymene)Ru(μ-Cl)Cl)2] and L1–L3. All the complexes were characterized by various spectroscopic and analytical methods. Complex 1 was also characterized by single crystal X-ray diffraction analysis, the results of which are in-line with the piano-stool structure elucidated from the spectroscopic methods. Cyclic voltammetry of 1–3 showed good stability of the Ru(II) state. The interactions of 1–3 with CT-DNA were investigated by UV-Vis studies and competitive binding with ethidium bromide (EthBr) using emission spectroscopy. Steady-state fluorescence quenching and synchronous fluorescence studies were performed to understand the interactions between 1–3 and bovine serum albumin (BSA). The geometry optimization of 1–3 was performed using the density functional theory (DFT). Complexes 1 and 2 were investigated for DNA binding using theoretical calculations. Molecule complexes 1-DNA and 2-DNA were huge (482–483 atoms, 2442 electrons) for any level of quantum chemical analysis. The semi-empirical PM6 method, which is more accurate than the force-field methods, was used, and the result suggested that several strong and weak electrostatic and intermolecular hydrogen bonding interactions exist between the outer edge of DNA and complexes 1 and 2. In addition, the in vitro cytotoxicity of L1–L3 and complexes 1–3 against a breast cancer cell line (MCF-7) was investigated by MTT cytotoxicity analyses. The studies performed here show that these complexes exhibit a good binding behavior with DNA and BSA and show good anticancer potency towards MCF-7 cells.


 Please wait while we load your content...
Please wait while we load your content...