Iodine-promoted one-pot synthesis of 1,3,4-oxadiazole scaffolds via sp3 C–H functionalization of azaarenes†
Abstract
An efficient iodine-mediated one-pot synthetic protocol for the synthesis of 2,5-disubstituted 1,3,4-oxadiazole scaffolds has been developed via sp3 C–H functionalization. Gratifyingly, this method involves oxidative amination with concomitant base-mediated cyclization of methylhetarenes and acylhydrazines by employing iodine and Cs2CO3. The key features of the present method include good functional group tolerance, a clean protocol, metal-free conditions and high yields, making this protocol an attractive strategy towards the synthesis of bioactive molecules and their key building blocks.
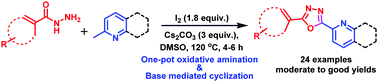


 Please wait while we load your content...
Please wait while we load your content...