Tetrathiopyridyl-tetrathiafulvalene-based Cd(ii) coordination polymers: one ligand, one metal cation, many possibilities†
Abstract
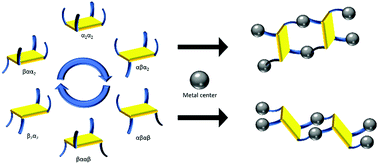
A novel tetrathiafulvalene (TTF) derivative bearing four peripheral 4-thiopyridyl units has been designed, synthesized and assembled with CdI2, Cd(NO3)2 and Cd(NCS)2 salts for the construction of a series of coordination polymers. Five networks presenting four different arrangements have been obtained and characterized by single-crystal X-Ray diffraction along with three isostructural compounds with other M(NCS)2 salts (M = Mn, Fe, Co). Whereas one is based on infinite {Cd(NCS)2}∞ chains, the others are constructed from mononuclear nodes with octahedral metal cations coordinated to four pyridyl groups of four different TTFs and to two anions in the axial positions. Interestingly, these coordination polymers built on structurally analogous metal nodes strongly differ in their overall organization (dimensionality, potential porosity, interpenetration), depending on the conformation adopted by the TTF bridging ligand in the crystal. Out of the six possible limit conformers for this derivative, four have been identified in the different materials described herein, highlighting the impact of using such a flexible ligand on exploring a broader landscape of possible crystalline architectures.


 Please wait while we load your content...
Please wait while we load your content...