Copper-catalyzed oxidative cleavage of Passerini and Ugi adducts in basic medium yielding α-ketoamides†
Abstract
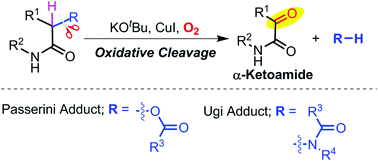
The aerobic oxidative cleavage of Passerini and Ugi adducts in the presence of base and copper(I) iodide is studied in detail. The oxidative cleavage yields α-ketoamides along with acids and amides from Passerini and Ugi adducts respectively. Mechanistic investigations revealed that the reaction proceeds via a radical pathway involving molecular oxygen. Control experiments with 18O-labeled Passerini adducts confirmed that molecular oxygen is the source of oxygen in α-ketoamides. A variety of Passerini and Ugi adducts were studied to explore the effect of substitution. Overall, the present study provides an insight into the reactivity of Passerini and Ugi adducts in strong basic conditions along with a method to prepare α-ketoamides.


 Please wait while we load your content...
Please wait while we load your content...