Binding of ellagic acid and urolithin metabolites to the CK2 protein, based on the ONIOM method and molecular docking calculations†
Abstract
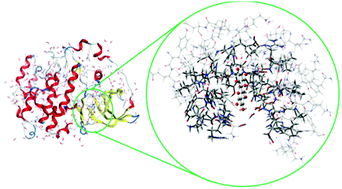
In order to explore changes in the inhibitory effect of ellagic acid (EA) under intestinal metabolism, twenty-eight ellagitannin derivatives and their metabolites, urolithins, were investigated in terms of their binding to the casein kinase 2 (CK2) protein. Molecular docking calculations reveal that all the considered ligands have a desirable potency to inhibit the CK2 protein. A rigorous three-layer ONIOM (M06-2X/6-31+G*:PM6:AMBER) method was also utilized to investigate the exact binding features and interaction details. Both hydrophobic and hydrophilic interactions are involved in the ligand–protein complexes, and the polarization effect is revealed as the predominant effect within the binding site. The water molecules in the active site improve the binding of the inhibitors to the CK2 protein via hydrogen bonding and lone pair–pi interactions. According to the ONIOM calculations, isourolithinA-3-glucuronide may be the most potent inhibitor against CK2 activity.


 Please wait while we load your content...
Please wait while we load your content...