Molecular modeling of cytotoxic activity of a new terpenoid-like bischalcone†
Abstract
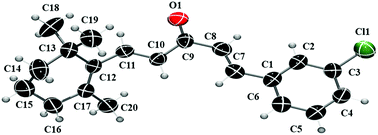
This study describes the synthesis and structure of (1E,4E)-1-(3-chlorophenyl)-5-(2,6,6-trimethylcyclohex-1-en-1-yl)penta-1,4-dien-3-one (BC I). X-ray single-crystal diffraction and Hirshfeld surface analysis describe supramolecular arrangement and topological analysis. Theoretical calculations, such as QTAIM, frontier molecular orbital, MEP and infrared spectra assignments, were performed at the B3LYP/6-311++G(d,p) level of theory. Also, this work evaluates molecular docking against DNA (PDB ID: 1BNA) and cytotoxic activity against two tumor cell lines. The BC I molecule has a half chair conformation of the cyclohexene ring, and the supramolecular arrangements are stabilized by C6–H6⋯O1 and C7–H7⋯O1 interactions. MEP and docking analyses indicate an electrophilic attack that is likely to occur on the carbonyl group.


 Please wait while we load your content...
Please wait while we load your content...