Facile synthesis of oligo anilines as permanent hair dyes: how chemical modifications impart colour and avoid toxicity†
Abstract
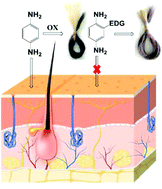
Many dyes for long-lasting hair coloring contain aromatic amines that undergo oxidative polymerizations, resulting in allergic contact dermatitis, with the potential to develop serious toxic effects. Among these amines, para-phenylenediamine (PPD) is a small molecule form of aniline commonly used in beauty products despite being a known allergen to humans. Hence, in this study we designed and synthesized safer PPD analogues through the synthesis of oligomeric PPD and the introduction of bulky side chains on PPD to overcome the PPD dye's toxicity. We hypothesized that (a) an increase in the molecular size of PPD by addition of the PPD monomer unit on free amines and (b) strategic functionalizations at the ortho position of PPD with strong electron-donating bulky groups are able to maintain the hair coloring properties, and increase the resistance to binding to skin proteins and therefore decrease the chance of skin sensitization. 13 oligomers were synthesized, with the aim to produce safer hair dyes while minimising eventual toxicity to humans. In particular, oligomers with bulky sidechains, PPD 6 (13), PPD 7 (14) and PPD 8 (15), displayed weak-to-moderate (27.1%, 24.1% and 34.0%) sensitization potential. The results confirmed the importance of having bulky and strong electron donating O-alkyl substituents in order to decrease the reactivity of PPD analogues towards skin proteins, thus preventing the interaction with immune cells and providing safer hair dyes.


 Please wait while we load your content...
Please wait while we load your content...