Oxidative addition of verdazyl halogenides to Pd(PPh3)4†
Abstract
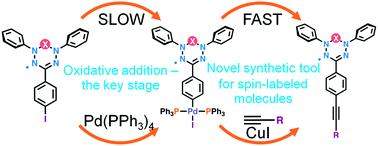
Direct oxidative addition of verdazyl halogenides to Pd(0) was studied and the role of this step in the Pd-catalyzed cross-coupling reactions was evaluated. A number of bis(triphenylphosphine)[verdazyl]palladium(II) iodide species were synthesized in yields of 89 to 92% and were shown to be persistent under ambient conditions. High synthetic potential of Pd-verdazyls was demonstrated by their efficiency and reactivity in the Sonogashira coupling reaction. These derivatives significantly expand the modern synthetic tools for development of spin-labeled materials and polyradical systems with desired functionalities.


 Please wait while we load your content...
Please wait while we load your content...