The α-alkylation of ketones with alcohols in pure water catalyzed by a water-soluble Cp*Ir complex bearing a functional ligand†
Abstract
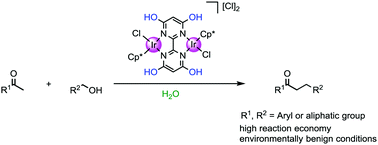
A water-soluble dinuclear Cp*Ir complex bearing 4,4′,6,6′-tetrahydroxy-2,2′-bipyrimidine as a bridging ligand was found to be a highly effective catalyst for the α-alkylation of ketones with alcohols in pure water. In the presence of catalyst (0.5 mol%), a series of desirable products were obtained with high reaction economy under environmentally benign conditions. The importance of the hydroxy group in the ligand for catalytic hydrogen transfer was confirmed by mechanism experiments. Furthermore, the application of this catalytic system for the synthesis of a biologically active molecule donepezil in pure water has been accomplished. Notably, this research would facilitate the progress of C–C bond-forming reactions in water catalyzed by water-soluble metal–ligand bifunctional catalysts.


 Please wait while we load your content...
Please wait while we load your content...