Exploring the hidden potential of a methoxy substituted HBT derivative as an efficient example of coupling of AIE and ESIPT processes and as an energy harvesting platform†
Abstract
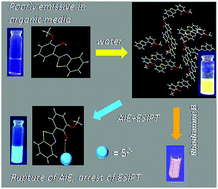
A methoxy substituted HBT derivative, namely 2-(benzo[d]thiazol-2-yl)-6-methoxyphenol (TMP), was synthesized and characterized. Detailed photophysical investigation of TMP indicated the coupling of excited state intramolecular proton transfer (ESIPT) and aggregation induced emission (AIE). Crystal structure analysis along with theoretical calculations on TMP showed equal preference of the proton to form hydrogen bonds with methoxy oxygen and the benzothiazole N atom, which is responsible for the observed AIE. TMP could be used as an efficient energy harvesting platform with rhodamine-B dye in aqueous solution, owing to Förster Resonance Energy Transfer (FRET) between the two. Finally, TMP can serve as an efficient probe for selective detection of sulfide in pure aqueous solution as well as in the solid state.


 Please wait while we load your content...
Please wait while we load your content...