The superior NO oxidation activity over La2CoMnO6 double perovskite to that of La2GaMnO6: effects of magnetic Co3+ and nonmagnetic Ga3+
Abstract
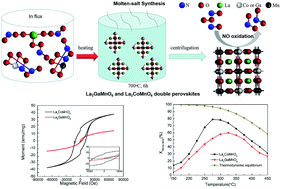
NO oxidation on transition metal-based perovskites has emerged as an effective strategy for reducing NOX in automotive and industrial exhaust. Magnetic Co3+ and nonmagnetic Ga3+ cations can change the magnetic properties of double perovskites, and also affect their oxidation activity with paramagnetic O2 and NO. Herein, La2GaMnO6 and La2CoMnO6 double perovskites with monoclinic structures were prepared via a facile molten-salt synthesis method. The coercive field (Hc), saturation magnetization (Bs), remanent magnetization (Br), and catalytic oxidation performance of La2CoMnO6 were obviously larger than those of the La2GaMnO6 double perovskites. Thus, it was concluded, the higher the magnetic properties, the better the catalytic oxidation performance over the double perovskites.


 Please wait while we load your content...
Please wait while we load your content...