Synthesis, structure and phenoxazinone synthase-like activity of three unprecedented alternating CoII–CoIII 1D chains†
Abstract
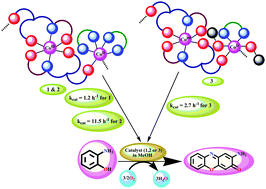
Three new one-dimensional mixed valence coordination polymers {[CoIII(L1)2][CoII(bpy)2]·ClO4·H2O}∞ (1), {[CoIII(L1)2][CoII(phen)2]·ClO4·H2O}∞ (2) and {[CoIII(L2)2][CoII(bpy)(CH3OH)2]·ClO4·H2O}∞ (3) (H2L1 = N-salicylidene-L-alanine, H2L2 = N-salicylidene-L-phenylalanine, bpy = 2,2′-bipyridine and phen = 1,10-phenanthroline) have been synthesized. Structural analysis reveals that the hexa-coordinated CoIII unit, [CoIII(L1)2]−, formed by two binegative tridentate Schiff base ligands contains uncoordinated oxygen atoms in the pendent carboxylate arm which further link the neighboring [CoII(bpy)2]2+ units to produce the μ1,3syn–anti carboxylato bridged one-dimensional (1D) coordination polymer in complex 1. Complexes 2 and 3 feature similar syn–anti carboxylato bridged one dimensional coordination polymers formed by joining of alternating CoII and CoIII units. Using o-aminophenol (OAPH) as the substrate, the phenoxazinone synthase-like activity of the complexes has been studied and the turnover numbers (kcat) have been calculated to be 1.2, 11.5, and 2.7 h−1 for 1–3, respectively. The mechanism of phenoxazinone synthase-like activity is proposed on the basis of mass spectral analysis, suggesting the cooperativity of CoII and CoIII centres, i.e., the CoII centre binds to the incoming substrate molecule, whereas the CoIII centre is involved in electron transfer from the substrate to molecular oxygen.


 Please wait while we load your content...
Please wait while we load your content...