Complexation of 2-thenoyltrifluoroacetone (HTTA) with trivalent f-cations in an ionic liquid: solvent extraction and spectroscopy studies†
Abstract
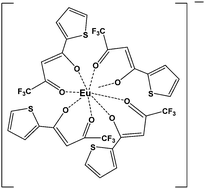
The complexation of 2-thenoyltrifluoroacetone (HTTA) with trivalent lanthanides was studied in a room temperature ionic liquid, viz. 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide (C4mim·Tf2N) by liquid–liquid extraction, absorption spectroscopy and time-resolved fluorescence spectroscopy. The metal ion distribution data indicated the extraction of anionic species of the type Ln(TTA)4−, where the charge balance of the C4mim·Tf2N phase is maintained by the transfer of one Tf2N− anion into the aqueous phase. Spectrophotometric titration of Nd3+ with HTTA indicated that the latter existed predominantly as the keto form in C4mim·Tf2N and did not form a complex with the metal ion. On the other hand, it formed complexes when TTA− salt was used where it predominantly existed in the enol form. Titration of Nd3+ with TTA− confirmed the formation of Nd(TTA)2+, Nd(TTA)2+, Nd(TTA)3° and Nd(TTA)4− species with the stability constant (log β) values of 5.82, 10.6, 14.5 and 17.8, respectively. Fluorescence measurements confirmed that the fully hydrated aqua Eu3+ cation in C4mim·Tf2N was completely dehydrated upon complexation with TTA−. Based on spectroscopic evidence, it was deduced that TTA− formed an 8 coordinated [Eu·(TTA)4]− anionic complex in C4mim·Tf2N.


 Please wait while we load your content...
Please wait while we load your content...