Vibrational spectra, hydrogen bonding interactions and chemical reactivity analysis of nicotinamide–citric acid cocrystals by an experimental and theoretical approach†
Abstract
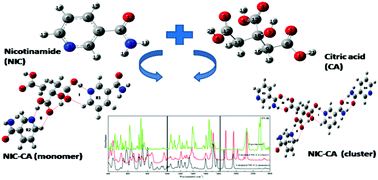
Nicotinamide (NIC), also called vitamin B-3, is commonly known as a pellagra-preventive drug. Citric acid (CA) is a weak tribasic acid, generally used as a flavouring and chelating agent. Herein, a combined experimental and quantum chemical approach was adopted to study the structural properties and spectroscopic signatures of nicotinamide–citric acid (NIC–CA) cocrystals using monomer (2NIC + CA) and cluster (4NIC + CA) models. In the cluster model, two additional NIC molecules were attached to cover the nearest possible interactions to understand the complete molecular geometries and hydrogen bonding interactions present in the cocrystal. In addition to this, our strategy was to calculate and analyse the physicochemical properties of NIC and CA along with improved properties after NIC–CA cocrystal formation. The observed red shift in the stretching modes of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H of the NH2 groups of NIC and the C
O and N–H of the NH2 groups of NIC and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H groups of CA along with the elongation in bond lengths in the cluster model of NIC–CA indicated the presence of hydrogen bonding interactions as well as the formation of cocrystals. Moreover, natural bond orbital (NBO) analysis was performed to obtain information about the interactions that were responsible for the stability and formation of the NIC–CA cocrystal. The ‘quantum theory of atoms in molecules’ (QTAIM) calculations revealed that all the intra- and intermolecular hydrogen bonding interactions present in the NIC–CA (monomer) and NIC–CA (cluster) model were partially covalent in nature. The molecular electrostatic potential (MESP) map of NIC and CA shows that the carbonyl (C
O and O–H groups of CA along with the elongation in bond lengths in the cluster model of NIC–CA indicated the presence of hydrogen bonding interactions as well as the formation of cocrystals. Moreover, natural bond orbital (NBO) analysis was performed to obtain information about the interactions that were responsible for the stability and formation of the NIC–CA cocrystal. The ‘quantum theory of atoms in molecules’ (QTAIM) calculations revealed that all the intra- and intermolecular hydrogen bonding interactions present in the NIC–CA (monomer) and NIC–CA (cluster) model were partially covalent in nature. The molecular electrostatic potential (MESP) map of NIC and CA shows that the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group and C–N of the pyridine ring in NIC are prone to electrophilic attack, and the hydroxyl (O–H) group of CA is prone to nucleophilic attack. The chemical reactivity parameters calculated using both models show that the NIC–CA cocrystal is more reactive and softer than NIC (API) and CA (co-former) since the band gap of the cocrystal is less than that of both NIC and CA.
O) group and C–N of the pyridine ring in NIC are prone to electrophilic attack, and the hydroxyl (O–H) group of CA is prone to nucleophilic attack. The chemical reactivity parameters calculated using both models show that the NIC–CA cocrystal is more reactive and softer than NIC (API) and CA (co-former) since the band gap of the cocrystal is less than that of both NIC and CA.


 Please wait while we load your content...
Please wait while we load your content...