Intramolecular hydrogen bond directed distribution of conformational populations in the derivatives of N′-benzylidenebenzohydrazide†
Abstract
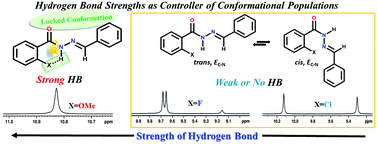
Extensive investigation by 1D and various 2D NMR techniques revealed the presence of only E isomers with respect to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and the existence of cis/trans conformations in the synthesized N′-benzylidenebenzohydrazide and its derivatives. The stable conformations of these molecules are attributed to the rotation of the molecular fragment around the C(O)–N bond. Interestingly, the conformational rigidity and the populations of the conformers are governed by the strengths of the intramolecular hydrogen bonds (HBs) between the ortho substituent on the benzoyl ring and the proton of the amide group, thereby permitting the architectural design of the preferred conformation of the molecules. The temperature perturbation studies and dilution studies using solvents of different polarities aided in the interpretation of inter- and intra-molecular HB interactions. The engagement of organic fluorine in the intramolecular HB is indubitably ascertained by the detection of the interaction strengths of a significant magnitude between organic fluorine and the NH proton, where the only mode of magnetization transfer between the interacting nuclei is HB (1hJFH). This is further endorsed by a physical parameter dependent perceivable variation in the strength of 1hJFH. The weak molecular interactions are further ascertained by DFT based computations.
N bond and the existence of cis/trans conformations in the synthesized N′-benzylidenebenzohydrazide and its derivatives. The stable conformations of these molecules are attributed to the rotation of the molecular fragment around the C(O)–N bond. Interestingly, the conformational rigidity and the populations of the conformers are governed by the strengths of the intramolecular hydrogen bonds (HBs) between the ortho substituent on the benzoyl ring and the proton of the amide group, thereby permitting the architectural design of the preferred conformation of the molecules. The temperature perturbation studies and dilution studies using solvents of different polarities aided in the interpretation of inter- and intra-molecular HB interactions. The engagement of organic fluorine in the intramolecular HB is indubitably ascertained by the detection of the interaction strengths of a significant magnitude between organic fluorine and the NH proton, where the only mode of magnetization transfer between the interacting nuclei is HB (1hJFH). This is further endorsed by a physical parameter dependent perceivable variation in the strength of 1hJFH. The weak molecular interactions are further ascertained by DFT based computations.


 Please wait while we load your content...
Please wait while we load your content...