Heteroleptic copper(i) halides with triphenylphosphine and acetylthiourea: synthesis, characterization and biological studies (experimental and molecular docking)†
Abstract
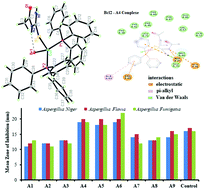
Nine new copper complexes with the general formula [CuX(TPP)n(ATU)3−n] (where X = Cl, Br, and I, ATU = acetylthiourea, TPP = triphenylphosphine and n varies as 0, 1 and 2) were synthesized in a simple fashion, by changing the ratio of the ligands. The synthesized complexes were characterized by techniques, such as FT-IR and NMR spectroscopy, CHNS elemental analysis and single crystal X-ray technique. The XRD technique showed the monodentate behavior of TPP and ATU. The synthesized compounds were utilized in different biological assays, which showed anti-bacterial, anti-fungal, anti-lieshmanial, anti-oxidant and cytotoxic properties against brine shrimps. In parallel, molecular docking was carried out to decipher the binding conformation and chemical interactions of the compounds in the active binding pockets of biological drug targets against bacterial pathogens and lieshmanial parasite, as well as a cancer target. All these analyses revealed compounds [CuCl(TPP)(ATU)2] and [CuI(TPP)(ATU)2] to be the most effective molecules of the series. Molecular docking indicated that hydrogen bonding and hydrophobic pi-interactions play important role for the activities of complexes with ligands TPP/ATU ratio of 1/2.


 Please wait while we load your content...
Please wait while we load your content...