Chemistry, chemical biology and photophysics of certain new chromene–triazole–coumarin triads as fluorescent inhibitors of CDK2 and CDK4 induced cancers†
Abstract
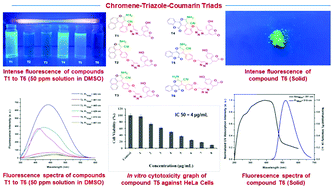
A new series of chromene–triazole–coumarin triads (T1 to T6) have been synthesized through the employment of a solvent-free mechanochemical multicomponent reaction, followed by a copper catalyzed (3+2) azide–alkyne cycloaddition (click chemistry). The molecules were investigated for their fluorescence and CDK induced anticancer properties. Half of the molecules (T1, T4, and T5) showed fluorescence in the solution state through ICT based electronic transitions, whereas the other half (T2, T3, and T6) showed solid-state fluorescence through aggregation induction. Computational studies on binding affinity revealed that all the molecules are generally selective towards CDK2 and CDK4. The in vitro biological activity studies showed that the molecules T2 and T5 exhibit potential as fluorescent inhibitors of CDK2/CDK4 induced tumors. T2 and T5 showed an IC50 of 7.5 μg mL−1 and 4 μg mL−1 respectively against the human cervical cancer cell line (HeLa).


 Please wait while we load your content...
Please wait while we load your content...