Amino-functionalized hypercrosslinked polymers for highly selective anionic dye removal and CO2/N2 separation†
Abstract
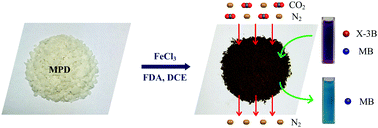
Recently, great improvements have been achieved in the fabrication of adsorbents. However, designing adsorbents with high adsorption capacity and superior selectivity still remains a big challenge. In the present study, a novel cost effective NH2-HCP adsorbent for toxic dyes and CO2/N2 separation was facilely synthesized through the one-step Friedel–Crafts alkylation reaction of m-phenylenediamine (MPD). The adsorption process under different adsorption parameters, namely, contact time and initial dye concentration were investigated in detail to explore the adsorption characteristics of the NH2-HCPs. Moreover, the experimental results demonstrated that the equilibrium adsorption isotherm and adsorption kinetics for anionic dye adsorption onto the NH2-HCPs fitted well with the Langmuir model and pseudo-second-order model, respectively. With a mesoporous structure and a high density of active amino groups, the amino-functionalized hypercrosslinked polymers (NH2-HCPs) not only showed an unprecedented adsorption capacity of 3290 mg g−1 for the anionic dye X-3B and almost no adsorption toward the cationic dye, but also possessed an excellent CO2/N2 selectivity of 122.


 Please wait while we load your content...
Please wait while we load your content...