Be2B6 and Be2B7+: two double aromatic inverse sandwich complexes with spin-triplet ground state†
Abstract
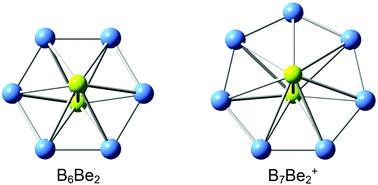
Quantum chemical calculations at both density functional theory (DFT) and single-point CCSD(T) levels suggest that Be2B6 (D6h, 3A1g) and Be2B7+ (D7h, 3A2′) clusters adopt interesting inverse sandwich structures with spin-triplet ground states, featuring a perfect planar B6 (or B7) monocyclic boron ring being sandwiched by two lone Be atoms. There is pronounced charge transfer from the Be atoms to the monocyclic boron rings, leading to [Be]2+[B6]4−[Be]2+ and [Be]2+[B7]3−[Be]2+ charge transfer complexes. Chemical bonding analysis showed that Be2B6 and Be2B7+ clusters have σ/π double aromaticity with 4σ and 6π delocalized electrons on the boron rings. The Be2B6 inverse sandwich possesses the smallest monocyclic boron ring motif in Be-doped boron clusters to date.


 Please wait while we load your content...
Please wait while we load your content...