A reversible and turn-on type fluorescence behaviour of hydrogen sulfide via a redox cycle between selenoxide and selenide†
Abstract
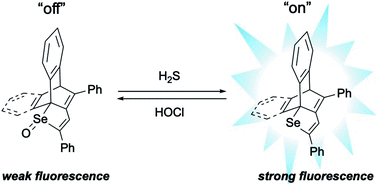
A turn-on type fluorescence behaviour of H2S based on a reversible selenoxide/selenide redox system is reported. Very weakly fluorescent selenoxides of 1,4-diphenyl-1-seleno-1,3-butadiene derivatives, incorporated with dibenzobarrelene (Dbb)- or (mono)benzobarrelene (Mbb) (DbbSeO and MbbSeO, respectively), are reduced quickly with NaSH as the equivalent of H2S in a dilute solution of CH3CN/PBS at pH 7.4 to yield the respective intensely fluorescent selenides (DbbSe and MbbSe). The selenoxides are highly selective for H2S compared to other biothiols such as L-cysteine (Cys) and reduced glutathione (GSH). On the other hand, the intense fluorescence of DbbSe is effectively quenched by oxidation with NaOCl as the equivalent of HOCl. The oxidation also showed high selectivity toward HOCl against other oxidants such as H2O2, t-BuOOH, and ONOO−. This reversibility of the fluorescence behaviour suggests that this system could monitor H2S and HOCl generation in one-pot. The fluorescence properties of DbbSe and DbbSeO were discussed based on the results of TD-DFT calculations.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...