New nitroindazolylacetonitriles: efficient synthetic access via vicarious nucleophilic substitution and tautomeric switching mediated by anions†
Abstract
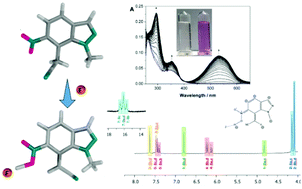
New N-alkyl-nitroindazolylacetonitriles were efficiently obtained via vicarious nucleophilic substitution of N-methyl-nitroindazoles with 4-chlorophenoxyacetonitrile. All compounds were fully characterized by NMR and mass spectroscopy techniques and the structures of some of them were additionally confirmed by X-ray diffraction analysis data. Tautomeric switching was observed in this series of nitroindazolylacetonitriles upon addition of basic anions followed by UV-Vis spectrophotometric and 1H-NMR titrations. The formation of tautomeric species induced by anionic species was endorsed by Density Functional Theory calculations.


 Please wait while we load your content...
Please wait while we load your content...