The reaction of phenoxatellurine with single-electron oxidizers revisited†
Abstract
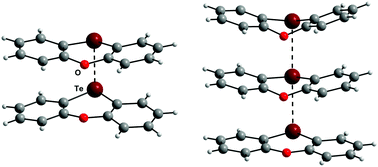
The reaction of phenoxatellurine (PT) with the known single-electron oxidizers AlCl3/CH2Cl2, [NO][SbF6] and [NO][BF4] provided the diamagnetic products [(PT)CH2Cl][AlCl4] (1), [PT2][SbF6]2 (2) and [PT3][BF4]2 (3), respectively, which were fully characterized by X-ray crystallography. The dications [PT2]2+ and [PT3]2+ present in 2 and 3 can be regarded as dimers of the elusive radical cation [PT]˙+ or as adduct between this dimer and neutral PT, respectively. The stacking between the aromatic layers of [PT2]2+ and [PT3]2+ can be best described using the pancake bonding (PB) model. The computational analysis uncovers the essential role of London dispersion effects for the stacking process and reveals the Te–Te interactions to be dominated by non-covalent bonding. The results are compared with those of the related thianthrene (TA) system.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...