Ruthenium-catalyzed deoxygenative hydroboration of carboxylic acids: a DFT mechanistic study†
Abstract
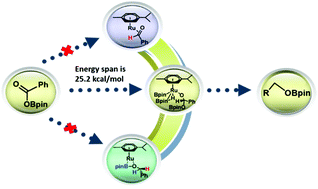
A density functional theory (DFT) study has been performed to investigate the mechanism of ruthenium-catalyzed deoxygenative hydroboration of carboxylic acid. The reduction of the carbonyl motif is suggested to proceed via an intramolecular 1,3-hydride shift mechanism. The second HBpin coordination intermediate with the Ru center could facilitate the pinBOBpin reductive elimination and the PhCH2OBpin reductive elimination step. The energy span for the reaction is 25.2 kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...